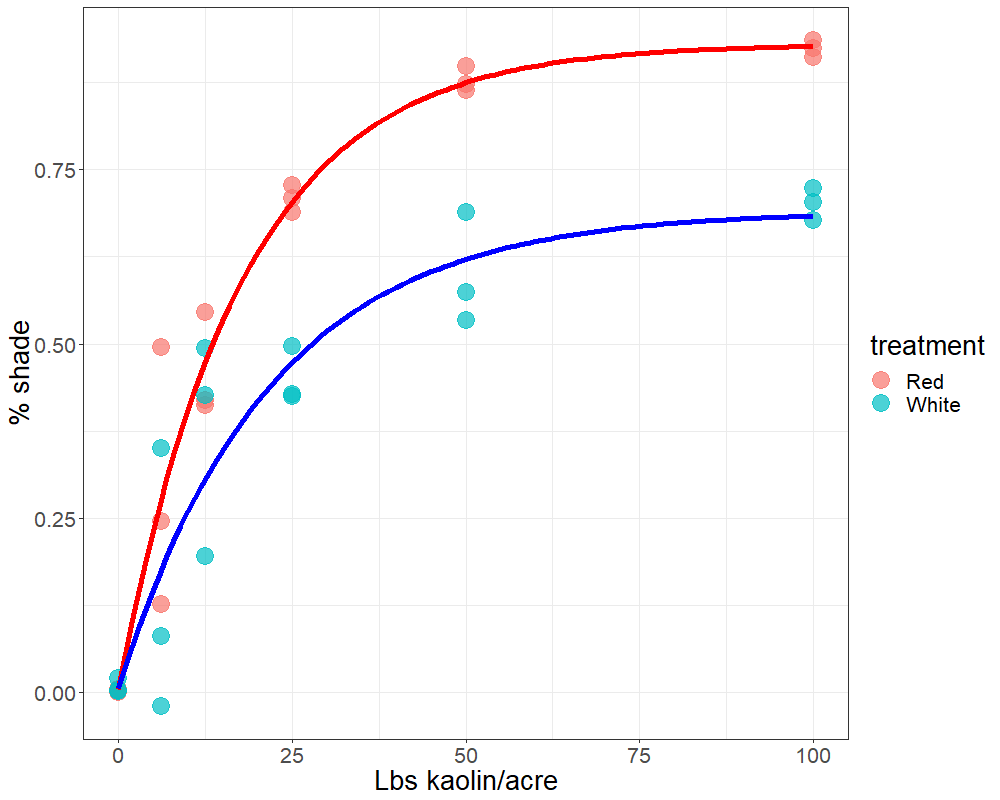
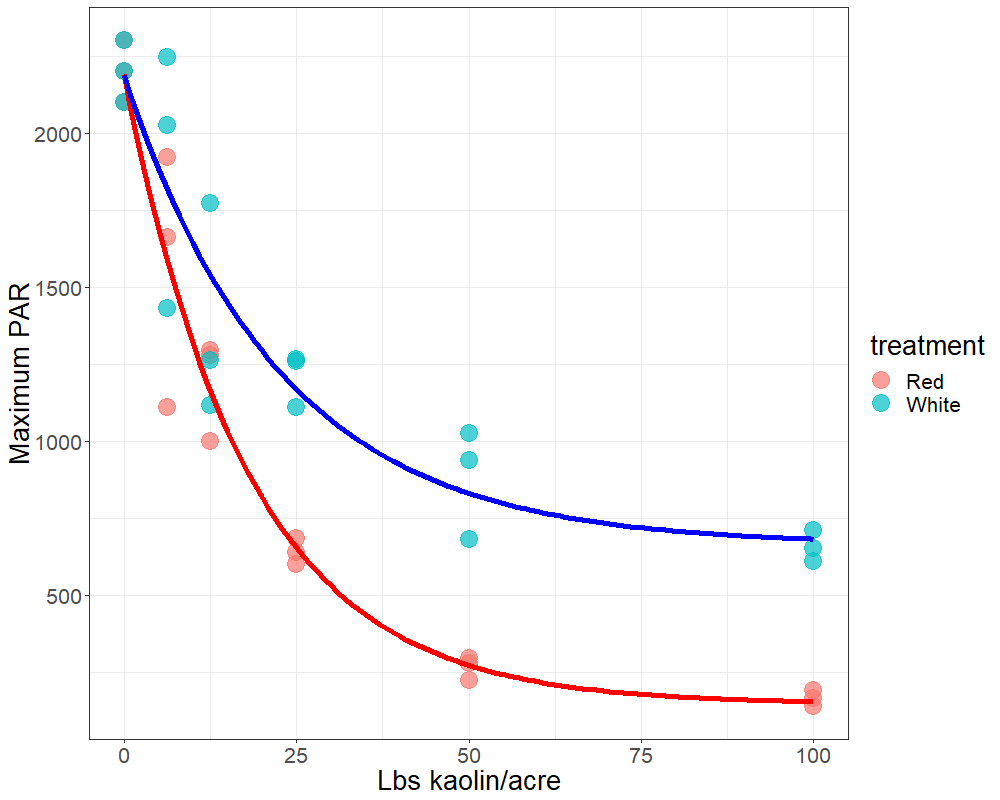
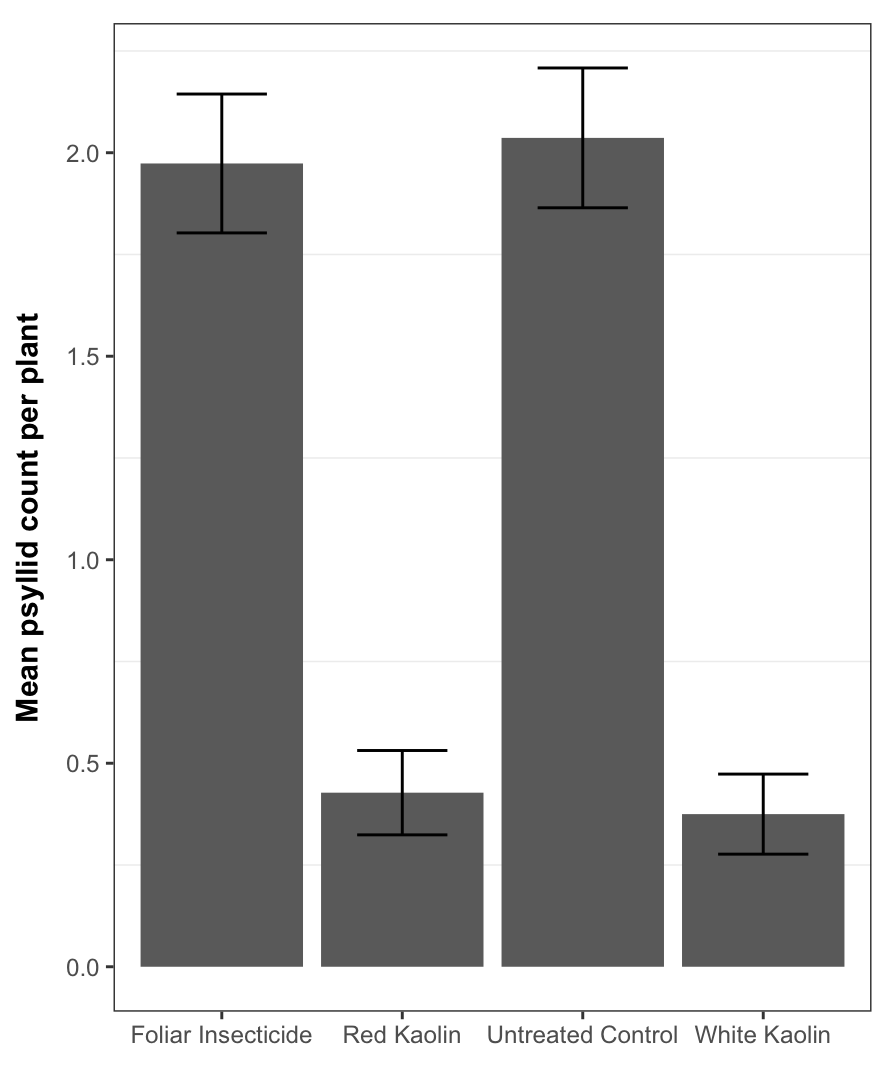
Plants face many hurdles to keep water moving up and sugars moving down, while keeping photosynthesis churning. Add to these challenges the fact that some plants may create their own limitations with constricted phloem. Recent work by a number of labs, in which we played a small part, shows that some trees may have restrictions in phloem movement.
Long leaf pines are rare in nature. Only a handful of conifer species worldwide have leaves longer than about 4″ (10 cm). Longleaf pines, though, native to the southeastern U.S. have needles up to about 1 foot (30 cm)! Why are long leaf species so rare, and why are our pine’s needles so long?
The challenge of narrow pipes
It turns out that most conifers, including longleaf pine (Pinus palustris), have narrow sets of phloem tubes that all the sugars from photosynthesis along the whole length have to move into in order to make it out of the leaf. This means that the leaf closer to the base is loading into the same narrow “pipes” that the tip is loading. Imagine if you took a bunch of bottles, lined them up and connected them with only small holes between them so that the each bottle drained into the next. Then you poured a liquid – imagine, say, pink lemonade, because that’s more fun – into all the bottles at the same time. The end bottle would drain the slowest, while the base bottle would drain fastest. The result is that, without some adaptation, the base and the tip compete for loading and the tip may be unable to add enough sugar to make the phloem flow. This may help explain why most species don’t have long needles. But that begs the question, how do longleaf pines do it?
Longleaf pines accept their limitations
Longleaf pines have anatomical adaptations that help reduce the loading limitation in their needles. They have specific files of phloem tubes that load mostly from certain leaf segments, which helps reduce the competitive effect, but not eliminate it. Because of this, they accumulate starch in their tips during the day, while the base regions run the sugar export show. But at night the tips rev up, and begin to export as the base begins to run out of reserves. In this way the various regions alternate in export over time, and keep export going.
Are long needles the only place where phloem constricts transport?
This is the clearest case of phloem transport limitation so far, but there may be other species that have similar challenges. Which we will discuss in the near future.